Butane Isomerization
reference information
Due to necessity of alkylate output increase on the Russian refineries, straight-run isobutane is not enough for this production supply. Taking into consideration isobutane consumption in MTBE and butyl rubber production, it becomes clear, that isobutane is a deficit product and must be replenished by normal butane isomerization.
On the modern alkylation units n-butane isomerization block is an element of the whole production and efficiency of that block determines, to a large extent, the whole process economy.
Process chemistry
It is assumed, that isomerization over bifunctional catalysts occurs through intermediate olefins. Olefin is generated on catalyst metal sites.
![]()
The reaction is reversible, and if a catalyst works at hydrocarbon high pressure, equilibrium shifts to the reverse side. But olefin is gotten bound by the catalyst acid function with formation of carbonium ion, and in spite of unfavourable equilibrium position, bigger amount of butane is transformed to olefin.
![]()
In the result of rearrangement:
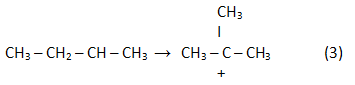
In the result of a reaction (2) reverse analogue isoolefin is generated:
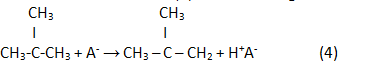
Finally, in the result of hydrogenation isoparaffin is generated:
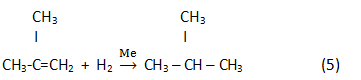
Process parameters
N-butane equilibrium isomerization reaction shifts to the side of isobutane generation upon temperature decrease (picture 1). Therefore the process becomes efficient only in case of temperatures below 250 оС.
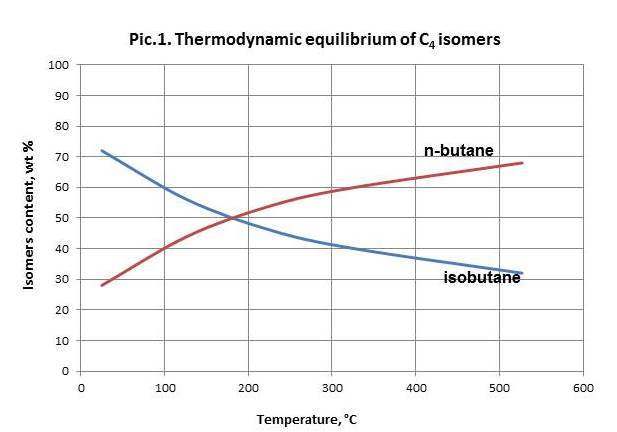
Space velocity decrease at permanent temperature and other conditions leads to decrease of isobutane content in a product.
Butane isomerization catalysts
Until recent times it was known, that only based on chlorinated aluminum oxide catalysts can work at this temperature area. Using this type of catalyst, UOP (USA) and British Petroleum developed processes, that got quite wide industrial application.
But catalysts, based on chlorinated aluminum oxide have several disadvantages:
- Permanent supply of chlorine-organic compounds for chlorine loss compensation during operation and related necessity of including caustic scrubber to the scheme for waste gases treatment.
- High sensitivity to sulfur, nitrogen and moisture impurities, that requires additional adsorptive after-treatment block besides the hydrotreating one.
- Activity decrease with operation and a short service life.
There are no listed disadvantages in the new type of isomerization catalysts, based on sulfated oxides.
Such oxide isomerization catalyst was developed by JSC SIE Neftehim in 2013 and was named SI-3. The base of the development is another oxide catalyst SI-2, intended for C5-C6 fractions isomerization and proven at 10 commercial units.
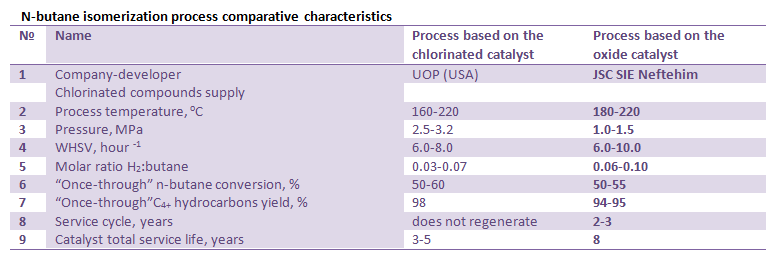
Main characteristics and parameters of n-butane isomerization process over SI-3 catalyst (JSC SIE Neftehim) as compared with the Butamer process (UOP) are shown in the table below.
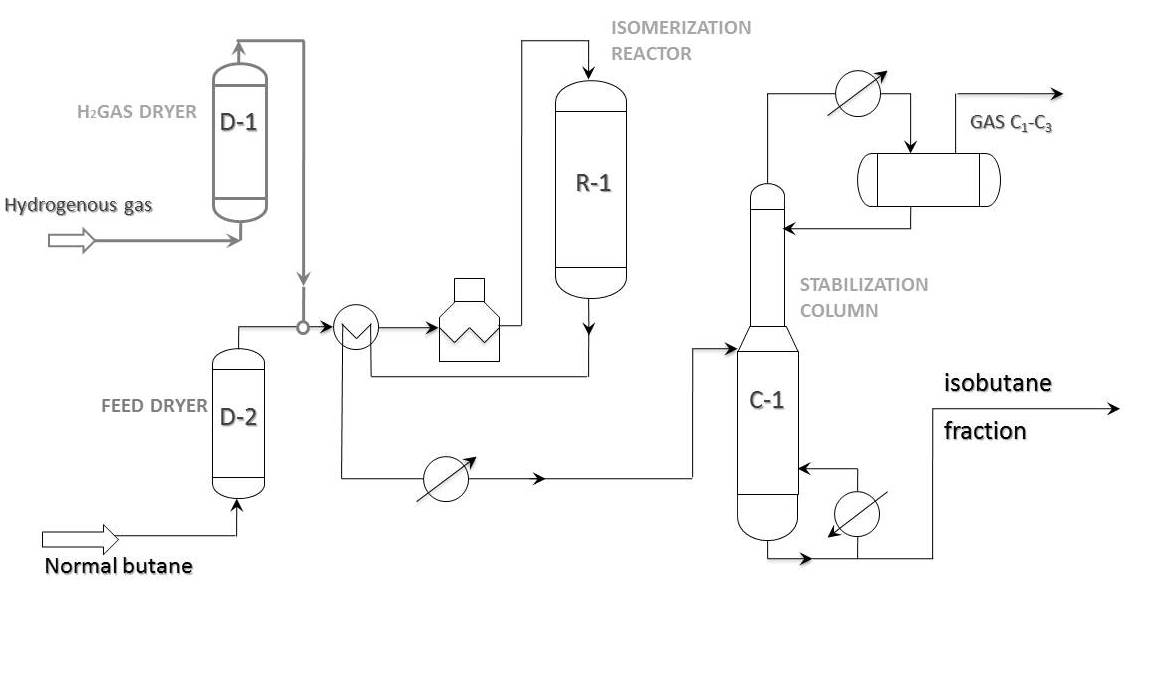
Process flow diagram
Principal process flow diagram of “once-through” n-butane isomerization is shown in the picture below. This scheme is widely used on butane isomerization units all over the world. As a rule, isobutane content in feed does not go beyond 20-25%. In case of 30% or more isobutane content in butane fraction, it is more profitable to pass the feed through deisobutanization column first. In this case the bottom product of the stabilization column goes to deisobutanization column.
Information of this chapter is given exclusively for a reference purpose. You can find information about SIE Neftehim, LLC's products and services in Developments and Services chapters.










